What is Safetac and how does it improve patient experience and outcomes?
As any clinician knows, patients can endure terrible pain when a dressing sticks to the wound or damages the skin. Likewise, a dressing that doesn’t protect the wound edges can cause maceration which slows down healing and can prolong suffering for those with chronic wounds.
We know that clinicians want to prevent this suffering and offer their patients more effective treatment options. That’s where Safetac comes in.
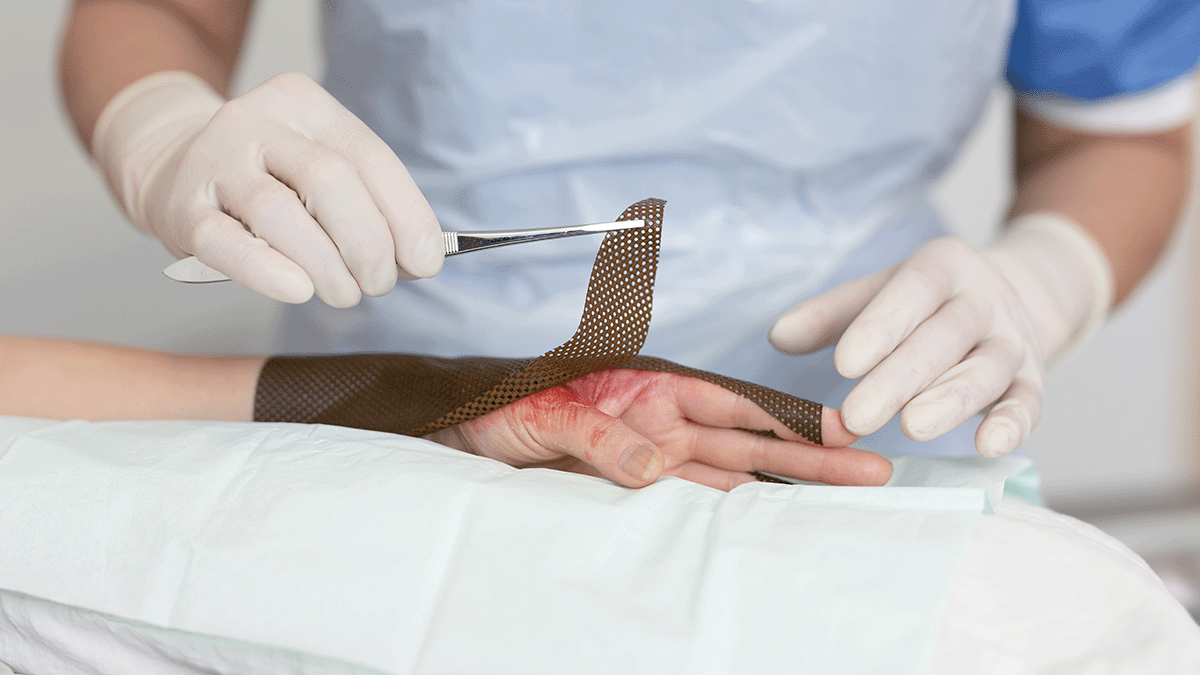
Safetac is the original game-changing contact layer. It is used across Mölnlycke’s advanced adhesive dressing ranges and facilitated the development of the first bordered silicone foam dressing. Safetac’s innovative design allows the dressing to mould softly to the skin and yet not stick to the moist wound.1-5 It will protect the wound edges to minimise risk of
maceration1, 4, 5, 7 and make the dressing easy to remove without damaging the skin.1-4
It all adds up to a less painful experience for patients at dressing change.1-6, 9 The combination of less pain and less skin and wound damage is the reason that numerous randomised controlled trials associate dressings with Safetac with faster wound healing.2, 3, 4, 9
Which dressings feature Safetac technology?
Since its launch at Mölnlycke, Safetac has been a key technology behind our most successful dressings. The range of dressings with Safetac has expanded over the years and there is now a foam dressing with Safetac for almost every type of wound:
- The Mepilex range with soft conformable foam is suitable for many chronic wounds. The newest member of this family, Mepilex Up, is designed especially for patients struggling with VLUs or similar exudating wounds.
- Mepilex Border offered revolutionary exudate management and is now superseded by the next-generation, uniquely conformable Mepilex Border Flex, with its increased ability to stay in place.
- Silver-infused dressings such as Mepilex Ag deliver gentle and effective burn care with an antimicrobial effect.
- Mepilex Border Sacrum and Mepilex Border Heel have extended Safetac’s influence beyond healing and into the prevention of pressure ulcers.
Other advanced dressings also feature Safetac:
- The Mepitel range, the first wound contact layers to incorporate Safetac, now includes Mepitel Film, a dressing uniquely proven to reduce radiotherapy-induced skin damage.8
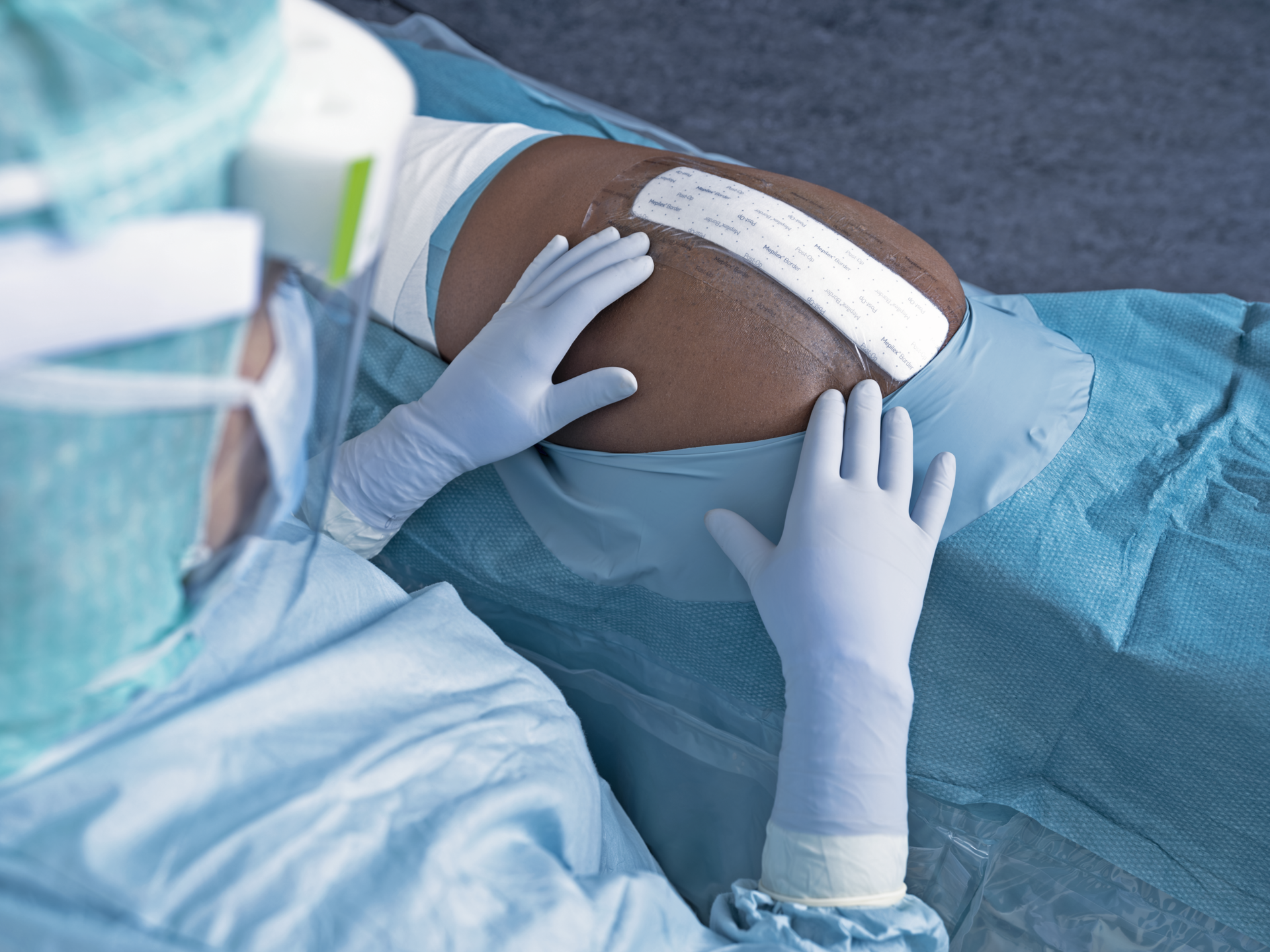
- Mepilex Border Post-Op, the advanced dressing for surgical incisions, was developed specifically to help reduce complications and support patients’ early mobilisation.1-6
How well-trusted is Safetac technology?
Since its launch, Safetac has been used around the world to improve the wound care experience of hundreds of millions of patients.
Safetac is trusted with good reason. At Mölnlycke, we are careful to support our products with robust evidence:
- more than 450 peer-reviewed findings confirm Safetac’s performance advantages.
- the evidence includes more than 30 randomised control studies.
What could Safetac silicone dressings mean for your patients – and your budget?
We know clinicians want to reduce patient suffering with less painful treatment options that support faster healing and improved outcomes. We also understand the pressures to reduce the cost of care.
In addition to improving the patient experience at dressing change, Safetac also:
- protects new tissue and intact skin so wounds are undisturbed, supporting faster healing 1-9
- seals wound margins to protect skin from leaks and maceration1, 4, 5, 7
By supporting faster healing, Safetac can not only improve the patient experience, it can also reduce the cost of treatment.2, 6, 9, 10
What is the story behind the development of Safetac technology?
Safetac was invented by a young product designer at Mölnlycke called Tomas Fabo at a time when advanced wound care technology was in its infancy. Tomas had seen at first hand the pain patients can experience during dressing changes and became driven by the need to prevent this suffering.
He began experimenting – even working at night in his own kitchen. Eventually he developed a type of silicone that was to become the key component of our early Mepitel range launched in 1989. Its promise was simple: it wouldn’t stick to the wound and it wouldn’t damage the skin and therefore, be less painful to remove.
The rest is history. Tomas achieved his aims and Safetac continues to be at the heart of our advanced dressing ranges, including our most recent innovation, Mepilex® Up.

How has Safetac changed the way we think about wound care?
Since the launch of Mepitel® in 1989, Mölnlycke has sold well over 4 billion dressings with Safetac, helping millions of people experience less painful healing and preventing hundreds of thousands of pressure ulcers.
Thanks to Safetac, wound care is now gentler, more effective, and more innovative than ever before.