Revolutionise care for people and planet
Transforming care for patients and caregivers
At Mölnlycke, we have always been dedicated to improving quality of life for patients and those who care for them. We do it by offering innovative, high-quality solutions for wound care and surgical procedures, and by helping you implement new, better ways of working. Ultimately, we enable safer, more efficient and more sustainable care. For people and planet.
-
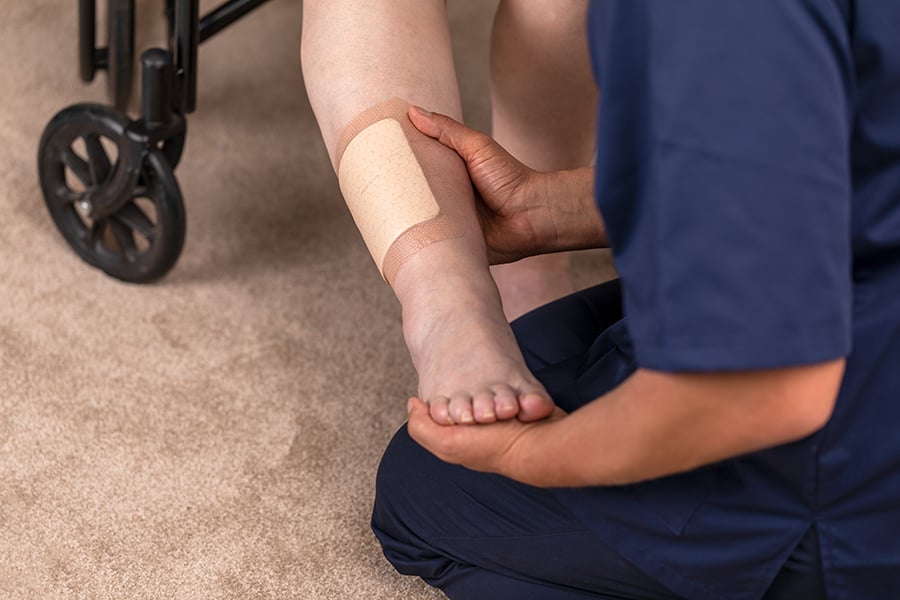
Wound care solutions
Explore our offer of innovative solutions that are optimised for health care workflows while improving quality of care for patients.
Discover our wound care solutions -
Surgical solutions
A safe and stress-free care environment, where clinicians can perform at their very best, doing the job they care most about.
Discover our surgical solutions
We're here to help
If you have any question about our products and solutions or need assistans, contact our sales team. We look forward to helping you find the perfect solution for your needs.
Contact usLatest updates
Discover our latest offers and campaigns.
Let's make room for what matters
There are many examples of non-value added tasks, in or around the OR, that could easily be eliminated, simply by implementing more efficient ways of working.
Read more-
Find the right antiseptic product
Our antiseptic products are gentle on the skin and effective on microbes with long lasting effect. Support skin integrity and reduce the potential of infections.
Read more -
Introducing the new Mepilex Up:
An innovative foam dressing designed for exuding wounds.
Read more

‘I always double-glove, and have from the very beginning. It's never been a challenge to work this way.’
David Revez Neurosurgeon
Latest news
Read all news-

Mölnlycke Health Care publishes Annual report for 2024
“Our strong financial position and continued growth allow us to direct our future and continued investments. At the same time, it is clear that our strategy and previous investments have paid off, enabling close relationships with our customers working alongside them to handle the challenges of healthcare today,” says Zlatko Rihter, CEO of Mölnlycke. Highlights from the year: Continued strong growth at 7.4% compared to previous year and annual sales of EUR 2,064 million with improved profitability. The progress in becoming a leader in sustainable healthcare was recognised with a platinum medal from EcoVadis. Focus on transformation through partnerships commenced in 2024 with the acquisition of P.G.F. Industry Solutions, the strategic partnership with Ondine Biomedical Inc and strategic investments in MediWound and Siren. The joint venture Tamer Mölnlycke Care in Saudi Arabia has started production and there are plans for expansion in India and China. Download and read the full report on www.molnlycke.com/corporate/about/reports For more information, please contact: [Contact card] Sofia Lindqvist
-
New study demonstrates the potential benefits of dressing regime improvements in delivering value-based chronic wound care
Barcelona, Spain. 27 March 2025. New study reports that switching patients with chronic wounds to the multi-layered, bordered silicone-coated foam dressing, Mepilex® Border Flex, alongside a clinician educational support programme, resulted in a significant reduction in the number of dressing changes being undertaken and a reduction in dressing-related costs.1 The study, sponsored by Mölnlycke Health Care, and conducted across primary care facilities in Seville, Spain was today presented as an e-poster at the 35th Conference of the European Wound Management Association (EWMA) 2025 in Barcelona, Spain by the principal investigator Dr Andres Roldan Valenzuela, Centro de Salud Mairena del Aljarafe – Cuidad Expo, Seville. The primary outcome of the study was a statistically significant reduction in the number of dressing changes undertaken, prior to and after the switch from a bordered foam dressing (baseline) to Mepilex® Border Flex; results demonstrated the median number of dressing changes reduced during the study period, from three in a seven-day period to just one.1 Other reported outcomes included:1 32% wounds healed by the final visit 50% reduction in wound area in four weeks in 75% of patients Two thirds (68.7%) reduction in wound area from baseline to the final visit Reduced pain at dressing changes (pain severity scores of 3.1 - 3.3 at baseline associated with previously used dressings, compared to scores of 0.5 - 1.1 recorded with Mepilex® Border Flex at the final visit) 44% reduction in weekly dressing cost per patient (5.38€ less after the dressing switch) No dressing related adverse events Almost all (97%) of the clinicians rated the overall performance of Mepilex® Border Flex to be better than the previously used dressings, while 100% of patients rated Mepilex® Border Flex as ‘good’ to ‘very good’ in terms of overall satisfaction, compared to 65% for the previously used dressings. Dr Andres Roldan Valenzuela, principal investigator commented “The results of this study clearly demonstrate how simple changes to a wound care treatment regime can bring about positive outcomes for both patients and healthcare providers. At a time when healthcare systems and staff are under increasing pressure on both cost and time it is encouraging that there are solutions to help drive efficiencies and improve outcomes for all involved.” For more information, please contact: [Contact card] Sofia Lindqvist About the study design The study involved 37 adult patients, aged between 30 and 90+ years presenting with wounds that had not reduced in size by >40-50% in the previous month and had been managed with foam dressing (other than Mepilex® Border Flex), for a minimum of four weeks (baseline). Category 2 pressure ulcers (24.3%) and venous leg ulcers (18.9%) were the most common wound types. Patients in the study had Mepilex® Border Flex applied to their wounds for ≥4 weeks, in conjunction with standard of care.
-

Mölnlycke Health Care expands investment in Tamer Mölnlycke Care joint venture
The Tamer Mölnlycke Care joint venture was initiated in 2021 with the agreement to develop a customised surgical procedure tray factory in Saudi Arabia, with first production expected in Q4 2024. The increased investment allows for an expansion in the range of Mölnlycke products manufactured and distributed in the country and to the neighbouring region. Adding drapes and gowns, emollient range and selected wound care products to the offering allows more healthcare professionals and patients in the region to benefit from Mölnlycke products and solutions. Zlatko Rihter, CEO, Mölnlycke Health Care comments “To ensure we meet customer demand, geographical expansion is a strategic priority for Mölnlycke Health Care. Increased investment in our existing long-term partnership with Tamer Group will importantly enable healthcare professionals and patients in Saudi Arabia access to our quality products and solutions helping to improve outcomes, and is a step closer to realising the potential of this rapidly growing healthcare market.” Speaking on behalf of Tamer Group, Chairman Ayman Tamer adds “This investment is key to enabling future growth in the region as outlined in the Saudi Vision 2030 - supporting the regional healthcare ecosystem, including exporting goods to the Middle East and Africa region, whilst developing local skills and expertise.” Mahmoud Wagih, CEO, Tamer Mölnlycke Care comments “I am delighted to lead this business where Mölnlycke and Tamer will build a strong, long-term successful joint venture, bringing superior health care solutions to our region.” The Tamer Mölnlycke Care joint venture is a long-term agreement between two organisations who have been working in partnership over the past decade; and are committed to meeting customer demand in the evolving healthcare landscape of Saudi Arabia and the wider region. For more information, please contact: Liz Neal Director of Communications Email: liz.neal@molnlycke.com Phone: +44 7787432560
-

Mölnlycke Health Care agrees to acquire leading wound cleansing manufacturer to consolidate its position as global leader in wound care
Gothenburg 12 April 2024: Mölnlycke, a world leading MedTech company specialised in wound care and wound management, announced today it signed an agreement to acquire P.G.F. Industry Solutions GmbH, the Austrian manufacturer of Granudacyn wound cleansing and moisturising solutions. Mölnlycke and P.G.F. have worked together for five years on the manufacturing and distribution of Granudacyn, a range of solution and gel products intended for use in the cleansing, irrigation and moisturisation of various wound types. The high quality and composition of Granudacyn ensures good biocompatibility, allowing it to be effectively used on a broad range of tissues in burns, as well as acute and chronic wounds. Mölnlycke currently distributes Granudacyn in over 50 countries around the world. “With an ageing population in combination with increased chronic wounds, such as diabetic foot ulcers or venous leg ulcers, it is our obligation to help ease the burden of wounds on national healthcare regimes” says Zlatko Rihter, CEO of Mölnlycke Health Care. “The acquisition of P.G.F. and its state-of-the-art manufacturing plant, will allow Mölnlycke to aggressively expand our Granudacyn business to improve the lives of even more patients.” “I’m excited for P.G.F. to become part of Mölnlycke and for the team to continue to manufacture products that support Mölnlycke’s Wound Care mission to help free patients from the burden of wounds” says Peter Fritz, P.G.F. founder and CEO, who will continue to lead this team and manufacturing process from P.G.F.’s Austria headquarters. Closure is expected after certain milestones are reached and will be communicated separately. For more information, please contact: Jennifer Doak Global Communications Director Wound Care Email: jennifer.doak@molnlycke.com Phone: +1 678 206 6179 About Mölnlycke® Mölnlycke Health Care is a world-leading MedTech company that specialises in innovative solutions for wound care and surgical procedures. Mölnlycke products and solutions are used daily by hospitals, health care providers and patients in over 100 countries around the world. Founded in 1849, Mölnlycke is owned by Investor AB and headquartered in Sweden. www.molnlycke.com About P.G.F. Industry Solutions P.G.F. Industry Solutions was founded in 2005 by Peter Fritz to develop and produce ecologically compatible hypochlorous acid based products to protect the user and their environment. The company is founded on more than 30 years of experience in research, development, production, and worldwide distribution of medical and measurement products. P.G.F. employs approximately 25 employees and is headquartered in Elixhausen, Austria. www.veriforte.com.
-

Mölnlycke signs a EUR 350 million revolving credit facility
The facility is backed by a group of seven leading global and regional banks, well diversified to match Mölnlycke’s geographical footprint. The banks are BNP, Danske Bank, HSBC, ING, JP Morgan, Nordea and SEB as mandated lead arrangers. SEB acted as the coordinator, documentation agent and facility agent for the transaction. For more information, please contact: Liz Neal Director of Communications Email: liz.neal@molnlycke.com Phone: +44 7787432560 About Mölnlycke Mölnlycke is a world-leading medical products and solutions company that equips healthcare professionals to achieve the best patient, clinical and economic outcomes. The core business is within the four Business Areas Wound Care, Operating Room Solutions (ORS), Gloves and Antiseptics. Mölnlycke employs around 8,700 people. The headquarters are in Gothenburg, Sweden, and the company operates in more than 100 countries worldwide. Mölnlycke is owned by Patricia Industries AB, which is part of Investor AB, an engaged owner of high-quality, global companies founded by the Wallenberg family in 1916. www.molnlycke.com
-

Mölnlycke Health Care announces launch of the 2025 Future Summit
“At Mölnlycke, we believe that education is a cornerstone of progress in healthcare. The 2025 Future Summit exemplifies our commitment to fostering innovation, enhancing clinical practice, and building partnerships that enable healthcare professionals to improve patient outcomes”, says Emma Wright, Chief Medical Officer at Mölnlycke Health Care. “We are thrilled to bring together such a diverse and accomplished group of experts to shape the future of wound care.” Offering a unique platform for collaboration Set to be a highlight of the professional education calendar, the 2025 Future Summit will host over 130 HCPs from vascular, orthopedic, general, and plastic surgery specialties. With the participation of global faculty leaders, the event will provide a unique platform to explore cutting-edge advancements, share best practices, and foster interdisciplinary collaboration in wound care and surgical excellence. The summit will take place on October 6-7, 2025, in the Netherlands and will be presided over by renowned experts Professor Javad Parvizi and Mr. Ibby Younis. “The Future Summit reflects the power of partnership and education in driving medical advancements,” says Professor Javad Parvizi, co-president of the summit. “By bringing together experts from diverse specialties, this event creates a unique opportunity to exchange knowledge and redefine standards in wound care and surgery.” A catalyst for innovation Attendees of the summit will be provided with the opportunity to engage in meaningful dialogue, access the latest clinical insights, and participate in interactive workshops led by global faculty leaders. “This summit is not just an event; it is a catalyst for innovation. It empowers clinicians from across EMEA to collaborate, learn, and apply transformative approaches that will ultimately improve patient care. Mölnlycke’s unwavering commitment to education is truly commendable,” says Mr. Ibby Younis, co-president of the summit. For more information, please contact: Sofia Lindqvist Media Relations Manager Email: sofia.lindqvist@molnlycke.com Phone: +46 72 255 35 09
-

Mölnlycke Health Care wins Swedish Sustainability Tech award
Gothenburg, Sweden. 26 November 2024. The award ceremony was held on 25 November at the Nobel Prize Museum in Stockholm, Sweden where Susanne Larsson, CFO & EVP IT, Digital Enablement, GBS and Indirect Procurement and Caterina Camerani, VP Sustainability at Mölnlycke, accepted the award on behalf of the company. “Our ambition is to transform our business to become a global leader in sustainable healthcare. This award is proof that we are on the right track. We are dedicated to equipping our customers with innovative and sustainable healthcare solutions – for our customers, our employees, our communities and the planet,” says Caterina Camerani, VP Sustainability at Mölnlycke. Mölnlycke ProcedurePak® trays enhance efficiency¹ and help decarbonise healthcare by reducing waste and greenhouse gas (GHG) emissions². ProcedurePak® offers a sterile tray with all necessary components, reducing setup time in the Operating Room by up to 40%¹, and packaging waste by up to 90%² compared to procedures conducted with single-packed products. In one tray package, hospitals can simplify procurement and help ensure patient safety³. The streamlined workflow decreases manual tasks, alleviating stress and freeing up more time for healthcare professionals to focus on patient care. Digital tools like the Mölnlycke Portal and CO2 calculator together with ProcedurePak® Value Report allow hospitals to quantify reductions in waste and GHG emissions, aligning with both sustainability and operational objectives. The judging panel commented: “In recognition of outstanding contributions to sustainability and innovation, this year’s Swedish award celebrates a project that has significantly enhanced operational efficiency while reducing environmental impact. Their commitment to sustainability is further demonstrated by reducing waste, CO2 emissions and preparation time while ensuring patient safety.” For more information, please contact: [Contact card] Sofia Lindqvist About the Cagemini Nordic Sustainability Tech Award The Capgemini Nordic Sustainability Tech Award celebrates organisations across the Nordic region creating innovative technology solutions for a sustainable future. The urgency of the global climate crisis demands ground-breaking technology and IT service innovations that scale across industries. The Nordic Sustainability Tech Award celebrates the inspirational tech pioneers pushing the boundaries of sustainable IT and their role in limiting the environmental and social impact of climate change. References 1 Greiling. A multinational case study to evaluate and quantify time-saving by using custom procedure trays for operation room efficiency. (poster from 2010). 2 Assessing the carbon and waste benefits of moving to Procedure Packs at Royal Liverpool and Broadgreen University Hospitals, NHS Trust, 2011. 3 Optimisation of perioperative procedural factors to reduce the risk of surgical site infection in patients undergoing surgery: a systematic review Key findings P. Calò , F. Catena, D. Corsaro, L. Costantini, F. Falez, B. Moretti, V. Parrinello, E. Romanini, A. Spinarelli, G. Vaccaro, F. Venneri. Discov Health Systems 2, 6 (2023). https://doi.org/10.1007/s44250-023-00019-9.
-

Mölnlycke Health Care appoints Guillaume Joucla as Chief Financial Officer
Gothenburg, Sweden. 25 November 2024. Guillaume Joucla has been appointed new Chief Financial Officer (CFO) at Mölnlycke Health Care. Guillaume Joucla will take office as CFO and member of Mölnlycke’s Executive Leadership Team, as of 1 January 2025. Guillaume Joucla most recently comes from the role of VP Global Finance & Strategic Projects Operating Room Solutions at Mölnlycke. He has also led global cross-functional transformational programmes and held General Manager positions of local operations. Guillaume Joucla wealth of experience from his extensive career within Mölnlycke has equipped him with deep insights into the company’s operations and strategic direction. “We are thrilled to welcome Guillaume to our executive team,” said Zlatko Rihter, CEO of Mölnlycke. “His leadership and expertise will be instrumental in driving our financial strategy and supporting our growth initiatives going forward.” Guillaume Joucla has a distinguished career; before joining Mölnlycke in 2008 he held senior management roles at ProxiMed Services and BioSteril. He holds a Postgraduate degree in Finance & Management from IAE Aix-Marseille Graduate School of Management, and a Master's degree in applied mathematics from Aix-Marseille University. “I am excited to continue my journey at Mölnlycke Health Care and contribute to our ongoing success,” said Guillaume Joucla. “I look forward to working with the talented team to build on Mölnlycke’s strong foundation and contribute to our future as we revolutionise care for people and planet.” Guillaume will assume the new position as of 1 January 2025 and will be taking over from Susanne Larsson, who is leaving Mölnlycke to pursue an opportunity outside of the organisation. -Ends- For more information, please contact: [Contact card] Sofia Lindqvist
-

Mölnlycke Health Care and Operation Smile help to change people's lives with newly inaugurated cleft care centre in Philippines
Gothenburg, Sweden. 19 November 2024. The Cebu Comprehensive Cleft Care Center of Excellence is now officially inaugurated. This milestone is a result of the longstanding partnership between Mölnlycke and Operation Smile – a global non-profit charity organisation. The centre will bring comprehensive care to people born with a cleft lip or palette (CLP) in the region and is expected to serve 10,000 patients in the first three years of its operations. The incidence of babies born with cleft lip and cleft palate is higher in South-East Asia than in other regions globally. The new clinic will offer life-changing surgeries and comprehensive cleft care to patients in the region where access to healthcare services is difficult. “The support from Mölnlycke in establishing the centre is grounded in our commitment to ensure equitable access to healthcare for all and the vision we have for achieving sustainable healthcare. A part of this vision is making the centre self-sufficient, and that includes raising the level of knowledge of the local healthcare professionals in the areas where Mölnlycke can offer expertise”, comments Eric de Kesel, COO & EVP Sustainability, Mölnlycke. “Cleft patients continue to be stigmatised and we are delighted to be able to contribute to changing their lives and fully including them in society,” he adds. “This clinic is a milestone for us, but also for cleft care globally, as it is also a training hub. In a rather lengthy construction project, our partner Mölnlycke has supported us financially while, in parallel with the construction, we have jointly created and rolled out a training programme with innovative methods and solutions,” says Emiliano Romano, Executive Director, Operation Smile Philippines. “Together with some of our volunteers, clinical specialists from Mölnlycke are training the local healthcare professionals in areas such as infection prevention.” The Cebu Comprehensive Cleft Care Center of Excellence is located at the Cebu City Medical Center (CCMC) hospital, in the Philippines. It is a 2,000 m2 state-of-the-art facility providing an optimal environment for delivering comprehensive CLP services, including speech therapy, dental and psychosocial care - in addition to the areas supporting the surgical flow, in alignment with the vision of surgical care that Mölnlycke and Operation Smile share. It is also fully manned by the local healthcare workers. -Ends- For more information, please contact: [Contact card] Sofia Lindqvist About Operation Smile Operation Smile is a leading global nonprofit bridging the gap in access to essential surgeries and health care, starting with cleft surgery and comprehensive care. We provide medical expertise, training, mentorship, research and care through our dedicated staff and volunteers around the world, working alongside local governments, nonprofits, and health systems, and supported by our generous donors and corporate partners. Visit Operation Smile for more information.
Upcoming events
See all eventsKnowledge
See all articles-
Antiseptics | 2 min read Preventing infections with the power of skin and nasal decolonisation
Mölnlycke is aware of Surgical Site Infections (SSI) and Healthcare Acquired Infection (HAI) occurring as adverse effects during hospital care. Combined approach for preoperative and postoperative patient bathing and nasal decolonization is required for consideration for healthcare professionals and their patients. When coordinated solutions are used like nasal antibiotics and skin decolonization risk of Hospital acquired S. aureus infections decreased by up to 60%. Here is how they work to reduce the risk of infections1. Skin decolonization Skin decolonization concerns the use of antiseptics solution to reduce the number of microorganisms on the skin. Common agents include for instance 4% Chlorhexidine Gluconate (CHG) and have a wide range of activity against gram positive and gram negative bacteria and yeasts, limited efficacy against viruses and fungi2. Accurate hand preparation for surgical procedure preformed by healthcare professionals and rationalizing whole body wash can reduce microbial load on the skin, thereby decrease the risk of infections. Nasal decolonization Nasal decolonization involves carriage of pathogens particularly Staphylococcus aureus including MRSA (Methicillin-resistant Staphylococcus aureus). Nose is a common reservoir for these bacteria, decolonizing the nasal passages can prevent the spread of pathogens to other parts of the body and to other individuals5. Using photodisinfection solution for this practice is particularly important in preventing Healthcare Acquired Infection and Surgical Site Infections and minimizing the risk of self-inoculations and cross-contaminations. The photodisinfection solution is not an antibiotic unlike other solutions that are used for this purpose. Photodisinfection is a safe and efficacious way to kill microorganism in the nose without creating antibiotic resistance and without compromise compliance. Skin integrity 4% Chlorhexidine Gluconate forms a protective layer to keep patients skin with reduced range of microorganisms, immediate effect after application and continually protected for hours after use3, 4. Mölnlycke designed antiseptic is dermatologically tested, with no colouring substances, no fragrances, no soya oil traces, minimize skin irritation and the potential for allergic reactions and increases users compliance. It also contains emollients and it is gentle on the skin even when used daily.
-

Gloves | 2 min read The dangers of powder in surgical gloves
Surgical glove powder can cause the following adverse health effects: Increased risk of surgical site infections (SSIs) Glove powder can trigger reduced resistance to infection, bacterial environmental contamination, foreign body reaction, delayed wound healing, adhesion formation and granuloma formation 1 2. All of these potential consequences can increase the risk of surgical site infection (SSI) 3. Latex allergy and occupational asthma Powdered latex gloves have been implicated as the largest single contributor to the latex aeroallergen levels in a healthcare facility 4 5. Latex proteins can be aerosolized by attaching to glove powder. This not only increases the risk of acquiring a latex allergy, but can also increase the risk of acquiring occupational asthma 6 . Glove powder increases latex allergy sensitization, potentially eliciting delayed hypersensitivity reactions. Powdered surgical gloves show higher levels of natural rubber latex allergens than gloves that are powder-free. This allows for the potential increase in latex sensitization and/or Type I reactions upon direct and indirect contact 7 8 9 10 11 Biogel surgical gloves: powder-free since 1984 Every single Biogel® surgical glove is powder-free, and has been for over 40 years. Biogel sold the world's first powder-free surgical glove in 1984, and over 40 years later, Biogel is still the only major surgical glove brand with an exclusively powder-free range.
-
Surgical | 4 min read One hospital achieves 37% more OR procedures using ProcedurePak®
As time management in healthcare, particularly in the surgical field, becomes more important, hospitals are actively looking for more efficiency from fewer resources. One hospital in France achieved significant improvements to operating room efficiency, according to a Mölnlycke ProcedurePak efficiency study. By implementing surgical procedure trays, European hospitals achieved 40-55% time savings in the operating room, making it possible to carry out more surgical procedures – around 37% more procedures in one hospital in France. [Video section] France Cathérine Laurent, OR nurse, Hôpital Privé des Peupliers, Groupe Générale de Santé, Paris, France Exploring how to improve operating room efficiency Hospitals in Europe are traditionally supplied with single-packed sterile products for surgical procedures. For each procedure, the nurses in the operating room need to source surgical instruments in the operating room and collect required materials from the storeroom unit by unit and set it all up for each procedure. Other necessary steps to get the right material in place include stock-taking and ordering, as well as warehousing and transportation. Hospital management today is striving for the most efficient treatment procedures without compromising on quality or safety. Thus there is increasing pressure to handle more interventions while relying on unchanged resources1, 2. Optimising internal processes to improve efficiency has become more and more important, and more effective operating room inventory management, down to the procedure level, has been identified as a way to achieve greater efficiency in the operating room. Study aim: Quantifying time savings from procedure trays The aim of the study was to investigate the effects of and quantify time savings when implementing ProcedurePak trays. Method This was an open prospective study performed as case studies in different hospitals located in Germany, France and Sweden. A total of 26 different ProcedurePak trays were used. For each of the hospitals the single-use material process was studied, from ordering of materials to disposal. This process typically consists of 6 main processes: internal order and delivery, receiving of goods in the surgical department, preparation and clean-up of surgery, external order, receiving of goods via the purchasing department and invoicing. The steps can be broken down into sub-processes and activities. For example, the main process – preparation and clean-up – can be broken down into 14 sub-processes or 33 activities. Each step in the process was described and measured both before and after implementation of the Mölnlycke ProcedurePak trays. For every step of the process, the time and cost drivers were identified by studying practical use and measuring time. Total annual time-saving after implementation of ProcedurePak trays in Germany. Results: Across hospitals, 40-59% time savings achieved The results of the study show that following the introduction of ProcedurePak trays, time-savings in the single-use material process were between 40% and 59% in the hospitals studied. The magnitude of the time-savings depends on several factors, including the number of different ProcedurePak trays used, number of components in each tray and the number of procedures performed with trays. The largest time savings were found in the preparation and clean-up of surgery, which is a key metric for operating room waste management initiatives. But time savings were also shown in other process steps. The time savings were used in different ways depending on individual objectives of the hospital, for example: Performance of additional surgical procedures, giving patients more timely treatment and maximising use of OR resources In the French hospital 37% more surgical procedures were performed following implementation. In the German hospital the number of interventions increased by 18% per year. Training of staff. Conclusions The case studies show that significant time-savings (40-59%) can be gained through implementing Mölnlycke customised procedure trays for surgical procedures in the operating room. The freed-up time makes it possible to perform additional surgical procedures. The financial effect of this is an area for further research. [Download] France case
-
Surgical | 2 min read All-in-one speciality drapes offer many benefits
Speciality drapes for surgery not only offer the infection control measures required in an operating room with their single-use nature but also meet the specific needs of particular surgical interventions. And with consideration for OR efficiency, surgical drape packs can add greater efficiency and time savings. Speciality surgical drapes for OR safety and efficiency Mölnlycke offer a comprehensive range of speciality drapes. The operating room is home to all different types of drapes, including universal sets, but intervention-specific all-in-one drapes have relevant features integrated in the drape for easy and efficient draping. Integrated pouches and tube holders to create an organised working field. Speciality drapes designed specifically for a given procedure, such as a knee operation or orthopaedic operation, offer coverage and protection where it is most needed, thereby reducing the risk of contamination. With fewer parts to handle, fewer steps to take and a shorter, more efficient set-up time, the patient is draped and ready for the procedure to begin faster. This contributes to OR safety and efficiency by: Making setup faster and more efficient, cutting down on prep and overall time in OR Single-use or disposable surgical drapes support aseptic practice Enabling more procedure-specific patient safety and ease of use for OR personnel Speciality drapes are designed to be optimally folded – the folding allows for aseptic application, easy for one person to drape. Allow for easy and efficient draping by one person For saving time – 3 times faster than draping with a universal drape solution¹ Fewer parts, fewer steps – for reducing the risk of contamination²
-
Surgical | 3 min read Calculate your CO2 to plot your sustainability journey
The last thing on your surgical team’s mind when hard at work in the OR is probably how much waste you are generating from single-use surgical instruments and drapes, or how much more efficiency you could gain from customised procedure-specific surgical trays. But as sustainability becomes a watchword for every industry, the single-use nature of antiseptic surgical environments is also coming under scrutiny. While patient safety leads as the most important consideration, hospitals continue to be beset by resource limitations balanced against new regulations that demand that they begin to operate more sustainably. Add to this equation the difficult-to-calculate environmental aspects of the operating room: from the CO2 expended in sourcing and producing the materials used in the production of OR goods to calculating the logistics of getting surgical trays and drapes and other surgical equipment into the hospital and properly distributed. But what if you could make your impact less opaque and gain clarity on exactly what impact the CO2 from your surgical business generates? Sustainable innovation Mölnlycke continuously innovates to offer the most sustainable solutions without compromising on the safety and quality of its products. By applying lifecycle thinking to existing products, the company proactively steers its product portfolios towards improved sustainability outcomes. An example includes a number of ISCC (International Sustainability and Carbon Certification) accredited sets of universal drapes offered by Mölnlycke. One of the three layers that make up the drapes is made of ISCC-certified renewable, biobased raw materials. As a result, an externally reviewed Life Cycle Assessment¹ showed these drapes to generate on average 20% less greenhouse gas emissions than traditional products. Guiding the conversion to sustainability Creating products that fulfil sustainability goals is one dimension of the issue. Another is ensuring that customers are aware of their existence and know the difference, providing clear guidance on a path toward conversion to more sustainable solutions. One such solution is a digital CO2 measurement tool that calculates the environmental benefits of choosing an ISCC drape over our standard product. Mölnlycke currently offers approximately 20 ISCC-certified drapes, consisting of up to 30% biobased material and a range of ISCC-certified surgical gowns. Demand for products using renewable, bio-based raw materials is particularly high in Scandinavia, the UK and Benelux, and is increasing in other markets as well. The calculator’s model includes the product’s entire life cycle, including Scope 1, 2 and 3.
-
Gloves | 2 min read Culture of OR safety: Double gloving for effective hand protection
Improving surgical gloving practice is about embracing a culture of safety and leading the OR team to the most effective hand protection.1 Communication is critical Preventive measures to reduce the risk of SSI, such as clean theatres, laminar air flow, good draping and skin prep, and so on, are protocol driven but rely very much on open communication policies in the OR and the surgeon setting that tone for the entire team. [Text & Image section] Nathal Communication into practice Ensuring safe OR practice also demands that gloves are donned properly, including double gloving and using a correct donning technique. The senior surgeon is responsible for staff, and should set the standard of practice for everyone in the OR. Reality: Glove barrier breaches Glove barrier breaches happen in the OR whether the injured party realises it or not. Sometimes only when removing surgical gloves does the wearer find that the gloves have been compromised. Double gloving helps protect the sterile inner glove – and hand – from bacterial contamination and keeps patient and OR staff safer, especially when an indicator glove system shows when the outer glove is breached.1 Reality: Tackling tactile sensitivity The surgical glove can affect the tactile sensitivity required, and, for Mr Coombs double gloving with high-quality surgical gloves in breast cancer surgery meant his tactile sensitivity was not affected compared to when single gloving when gripping and working with breast tissue. For surgeons, their hands are everything. Double gloving offers an efficient, cost-effective way to add that extra layer of protection during surgical interventions.2, 3 Contact us to find out more Visit Clinical Learning Hub
-
Surgical | 1 min read 10% more surgeries performed in Belgium
[video section] case berlin Dr Maurice Mommaerts, Maxillofacial Surgeon AZ Sint-Jan Bruges-Ostend hospital It has increased its number of interventions by 10% each year. The staff save time. They also comment on a more pleasant work environment and better patient care. AZ Sint-Jan Bruges-Ostend Av Maxillofacial Surgeon Dr Maurice Mommaerts said: ‘Mölnlycke Procedure trays contribute to a pleasant work environment, less stress and better use of time – in one word, efficiency.’ Director General Dr Hans Rigauts said: ‘Both nurses and doctors who use Mölnlycke Procedure Trays are clearly satisfied with them.’ [Download] case
-

Surgical | 1 min read Tray changes increase procedures by 17% in Finland
[Video section] Finland case Tarmo Martikainen, CEO at Coxa Hospital, Finland Coxa increased the number of procedures performed by 17 percent and incorporated lean processes into its daily work. [download] reference
-
Gloves | 1 min read The new generation of (NRL) surgical gloves
Different materials like natural rubber latex used in manufacturing of medical and surgical gloves have had unintended consequences – but should NRL be reconsidered? Materials like natural rubber latex (NRL) have been a lightning rod for their impact on individuals with latex allergies. But knowing what we know now about NRL, is it time for a re-appraisal? What does the future of glove manufacture look like, and can – and should – NRL make a comeback? [Text and image section] Haydn
