Mölnlycke Health Care is a world-leading MedTech company that specialises in innovative solutions for wound care and surgical procedures. Mölnlycke products and solutions are used daily by hospitals, health care providers and patients in over 100 countries around the world. Founded in 1849, Mölnlycke is owned by Investor AB and headquartered in Sweden. www.molnlycke.com
Media room
Press releases
-

Media room: Mölnlycke Brunswick, Maine site expansion
-

Mölnlycke Health Care breaks ground on $135 million expansion of Wound Care manufacturing facility in Brunswick, Maine
Brunswick, Maine, USA and Gothenburg, Sweden. 23 September 2025. Mölnlycke® Health Care, a global leader in MedTech, headquartered in Gothenburg, Sweden, today celebrates the groundbreaking of a major expansion to its wound care manufacturing capacity in Brunswick, Maine. This milestone marks the beginning of a $135 million investment aimed at significantly increasing Mölnlycke’s manufacturing capacity in the United States.
-
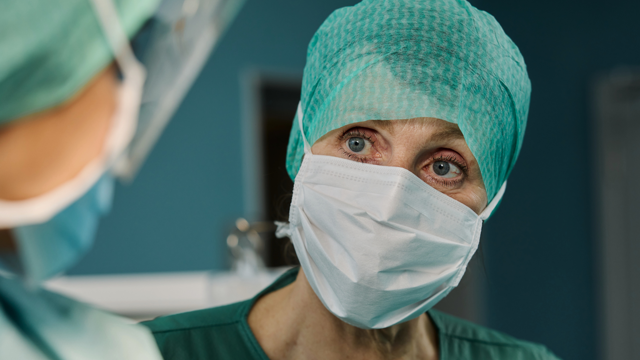
High burnout among operating room staff poses significant risk to patient safety—new review reveals
Gothenburg, Sweden. 17 September 2025. A new narrative review published in the current issue of Journal of Patient Safety, titled ’Putting Patients at Risk: The Effect of Health Care Provider Burnout on Patient Care in the Operating Room’ reveals alarmingly high levels of burnout among healthcare workers in operating rooms (ORs) and warns of the serious implications for staff well-being, care quality and ultimately patient safety.
-

Mölnlycke awarded second consecutive EcoVadis Platinum medal for sustainability performance
Gothenburg, Sweden. 21 August 2025. Mölnlycke®, a world-leading MedTech company, has once again been recognised as a global sustainability leader, earning the prestigious EcoVadis Platinum medal for the second year in a row.
About Mölnlycke
Press contact

Jamie Smith
Media Relations
Email: jamie.smith@molnlycke.com
Phone: +46 723 757507